Testimony to the House Energy and Commerce Subcommittee on Health Regarding Seniors’ Access to Breakthrough Medical Technologies
Contents
Transformative New Technologies Revolutionizing Cancer Screening. 6
The Importance of Early Cancer Detection. 7
Specific Benefits of MCED Screening Technologies 10
Addressing Potential Misgivings About MCED Approaches 12
An Emerging and Intensely Competitive Global Industry 12
Getting the Regulatory and Coverage Environment Right for MCED in the United States 13
Multi-Cancer Early Detection Policy Recommendations 15
Supporting Innovation in Breakthrough Medical Products 15
Introduction and Summary
The Information Technology and Innovation Foundation (ITIF) is pleased to submit these comments in response to the House of Representatives Committee on Energy and Commerce Subcommittee on Health hearing on “Examining Policies to Enhance Seniors’ Access to Breakthrough Medical Technologies.” Founded in 2006, ITIF is an independent 501(c)(3) nonprofit, nonpartisan research and educational institute that has been recognized repeatedly as the world’s leading think tank for science and technology policy.
Cancer remains a global scourge, the second-leading cause of death among Americans, killing over 600,000 annually. About 40 percent of Americans will develop cancer in their lifetimes. Age is the leading risk factor for cancer; therefore, it’s not surprising the disease hits America’s older citizens particularly hard. Americans over 65 account for 60 percent of newly diagnosed malignancies and 70 percent of all cancer deaths. 1,200 Medicare beneficiaries are diagnosed every day with late-stage cancer. That’s 50 Medicare beneficiaries an hour.
Unfortunately, despite some progress, too many cancers are still found late—when outcomes are worse and costs are highest. That’s in part because it’s remained extremely difficult to effectively preventively screen for cancer at the population scale. In fact, only five types of cancer—breast, cervical, colorectal, prostate, and “high-risk” lung—currently have guideline-recommended screening options available. The vast majority of cancers—including blood, head and neck, pancreatic, ovarian, and liver cancers, among others—have no guideline-recommended screening tests available. In fact, 70 percent of all U.S. cancer fatalities come from cancers for which there are currently no proven screening tests. This explains why so many cancers are unfortunately detected only when patients arrive at doctors’ offices manifesting physical symptoms; as only 20 percent of U.S. cancer cases are screen-detected.
It’s thus time for the United States (and the world) to embrace a revolutionary new approach to cancer detection, akin to major technological advancements that have resulted in vast improvements for Americans’ health and economic well being. Enter multi-cancer early detection (MCED) screening tests, which, from a simple blood draw, can detect as many as 50 different kinds of cancer with a low false positive rate and connect the cancer to the tissue of origin in the body. MCED tests use modern technologies including artificial intelligence (AI), machine learning, and genomic sequencing to identify circulating tumor DNA (ctDNA) shed by cancer cells in the body. MCED tests herald the ability to test for many cancers simultaneously and also to detect cancers at early stages of progression in the body.
Detecting cancers earlier could produce tremendous individual health, public health, and economic benefits. Catching cancers earlier can give patients a four times greater chance of survival. Indeed, when cancer is diagnosed after it has spread, the five-year cancer-specific survival rate is 21 percent, compared with 89 percent when the cancer is diagnosed early and still localized. Likewise, cancers are far more economical to treat at earlier than later stages of disease progression. In the Medicare population, average total annual costs of care are up to seven times higher for Medicare beneficiaries who are diagnosed at later stages rather than earlier stages. In short, MCED technologies can detect more cancer earlier, saving lives and saving costs.
The United States has led in the development of MCED screening tests, but competitors are emerging worldwide. In particular, China is prioritizing the development of MCEDs as part of its national priority to dramatically expand its global biotech leadership. If America is going to continue to lead in this field amidst growing global competition, enabling both its innovative companies to thrive and its citizens to enjoy the benefits of these technologies, policymakers will need to craft a supportive regulatory and coverage environment. If we don’t, other countries will, and they may eventually come to dominate the largest longitudinal public health application of genomic sequencing.
Congress has a long history of acting to create Medicare coverage for cancer screening, most recently when the Balanced Budget Act of 1997 added prostate and colorectal cancer screening benefits to Medicare coverage. To help make it possible for older Americans to take advantage of the enormous potential of MCED technologies, it’s time for Congress to act again. Congress should pass the bipartisan “Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act,” which would create the authority for the Centers for Medicare & Medicaid Services (CMS) to use an evidence-based process to cover blood-based MCED tests and future test methods once approved by the U.S. Food and Drug Administration (FDA), while maintaining CMS’s authority to use an evidence-based process to determine coverage parameters for these new types of tests. The legislation affirms that multi-cancer detection tests are designed to complement, not replace, existing screening cancer screening methods. The bipartisan legislation enjoys the sponsorship of 295 members of the House of Representatives and 62 Senators as of this hearing date.
In summary, MCED technologies are poised to radically transform America’s cancer-screening paradigm for the significant benefit of Americans’ individual and public health. Congress can play a critical role in making that transformation happen.
The Scourge of Cancer
Cancer remains one of humanity’s most implacable enemies, with over 22 million cases diagnosed globally in 2022 and 35 million cases expected annually across the world by 2035.[1] Cancer is responsible for almost one in six deaths globally.[2] In the United States, cancer claims over 600,000 lives annually (an estimated 612,000 in 2024), making it the second-most common cause of death, and which is expected to become the leading cause by 2030.[3] Over 1,600 Americans perish each day as a result of cancer.[4] Approximately one in four Americans (40 out of 100 men and 39 out of 100 women) will develop cancer during their lifetimes.[5]
Cancer hits America’s older citizens particularly hard. Age is the leading risk factor for developing cancer. The median age for a cancer diagnosis is age 66.[6] Eighty-eight percent of individuals diagnosed with cancer in the United States are age 50 or older, while 57 percent are age 65 or over.[7] Citizens over 65 account for 70 percent of all cancer deaths. Over 1,200 Medicare beneficiaries are diagnosed every day with late-stage cancer (that’s 50 an hour). Approximately 1 million Medicare beneficiaries were diagnosed with cancer in 2024.[8]
America’s Cancer Fight
Fortunately, American cancer fatality rates have decreased somewhat over the past half-century, largely the result of a combination of more-effective screening approaches, an overall decrease in smoking in the population, and more-effective treatment and therapeutic options. For some of the most common cancers—lung, colorectal, breast, and prostate—reductions in smoking and improvements in screening have led to 36 percent fewer deaths than would have occurred otherwise.[9] Americans’ deaths from cancer have fallen from 193.9 per 100,000 population in 1950 to 152.5 by 2017.[10] Since peaking in the early 1990s (at 215 per 100,000 population), U.S. cancer death rates have declined by 27 percent.[11] This decline translated into more than 2.9 million fewer cancer deaths from 1991 to 2017.[12] And breakthrough therapies such as Avastin and Herceptin for breast cancer, Keytruda for lung cancer, and Yervoy for melanoma help explain why American citizens enjoy the highest cancer survival rates in the world. For instance, over 99 percent of U.S. women suffering from localized breast cancer are still living five years later.[13] One study estimates that approximately 73 percent of survival gains in cancer are attributable to new treatments, including medicines.[14] Moreover, as Lichtenberg explains, “During the period 2000–2011, the premature (before age 75) cancer mortality rate … declined by about 9 percent.… In the absence of pharmaceutical innovation during the period 1985–1996, the premature cancer mortality rate would have increased about 12 percent during the period 2000–2011.”[15]
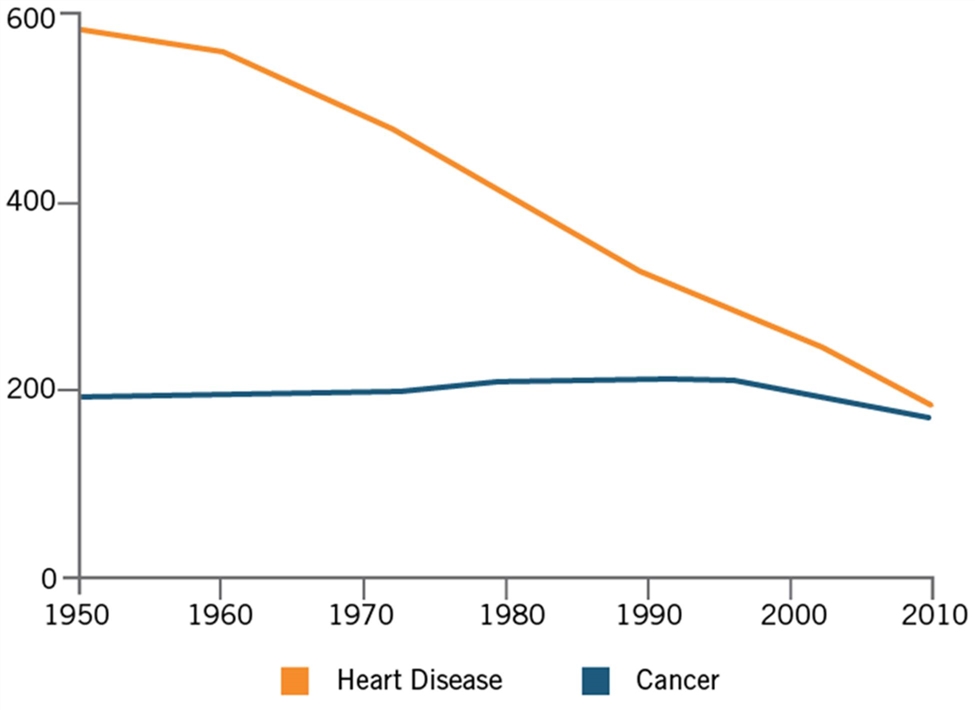
Yet progress, while real, has been slow. As Azra Raza, a professor of medicine and director of the MDS Center at Columbia University, writes, “Cancer is still beating us … I have been studying and treating cancer for 35 years, and here’s what I know about the progress made in that time: There has been far less than it appears.”[16] She points out that, for all the progress, overall cancer death rates are not dramatically different from what they were in the 1930s, before they began increasing alongside the rise in smoking. Indeed, while the age-adjusted death rate per 100,000 U.S. population from heart disease fell by roughly two-thirds from 1950 to 2010, the similar rate for cancer just barely decreased. (See Figure 1.) As Dr. Bert Vogelstein, a professor of oncology at Johns Hopkins University, explains, part of this disparity can be attributed to the fact that the heart disease research community has largely focused on early detection and prevention, “whereas the oncology community has been more focused on curing advanced disease.”[17] Or, as Raza puts it, “We now invest a lot of effort into finding minimal residual disease. Why not apply the same rigor and focus to finding minimal initial disease?”[18]
Figure 1: Age-adjusted rate of death per 100,000 population[19]
The Economic Cost of Cancer
Thus, despite some progress, cancer still afflicts millions annually and imposes tremendous costs on the U.S. health care system, as well as the broader economy. Cancer is the second-most-costly disease in the United States.[20] National cancer costs were estimated to be $208.9 billion in 2020.[21] By 2030, America’s cancer care costs are expected to rise to $246 billion (a 34 percent increase from the year 2015).[22] Globally, the economic cost of cancer care is expected to total $5.3 trillion between 2020 and 2050 (and that’s just for 29 cancers).[23]
A 2008 study estimated that the economic value of life lost from all cancer deaths in the year 2000 totaled $960.6 billion and predicted that the total value of life lost in 2020 from cancer deaths in the United States would reach $1.5 trillion.[24] The tremendous costs cancer imposes conversely suggest tremendous benefits if cancers could be detected earlier when treatments are more likely to succeed and as more-effective treatments and therapeutics for cancer are invented. In fact, one study finds that cancer screenings have already saved the United States at least $6.5 trillion.[25] Further, Murphy and Topel, considering the benefits of increased longevity and improved quality of life, find that a 1 percent reduction in mortality from cancer could deliver roughly $500 billion in net present benefits, while a cure (if one could be achieved) could deliver $50 trillion in present and future benefits.[26]
The number one risk factor for cancer is age. And, today, over one-third of U.S. cancer costs are attributed to the Medicare population.[27] Meanwhile, analysts estimate that Medicare will spend $1 trillion on cancer over the next decade.[28] Further, in the Medicare population, average total annual costs of care are up to seven times higher for Medicare beneficiaries who are diagnosed in later stages as opposed to earlier stages of the disease.[29]
Transformative New Technologies Revolutionizing Cancer Screening
Cancer will not be beaten without hard-fought biomedical innovation, both in the development of technologies to screen for cancers and therapeutics to treat them. However, only five types of cancer—breast, cervical, colorectal, prostate, and “high-risk” lung (collectively accounting for about 40 percent of U.S. cancer incidence)—currently have guideline-recommended screening options available today. This means that the vast majority of cancers—including blood, head and neck, pancreatic, ovarian, and liver cancers—have no guideline-recommended screening tests available.[30] Seventy percent of U.S. cancer deaths occur from cancers for which there are no guideline-recommended screening alternatives.[31] As such, the vast majority of cancers are unfortunately found only when patients arrive at doctors’ offices manifesting physical symptoms. Only 14 percent of cancers are found through the guideline-approved screenings for the five types of aforementioned cancers.[32]
Fortunately, a revolutionary new approach called multi-cancer early detection (MCED)—powered by a suite of advanced technologies including artificial intelligence, machine learning, and gene sequencing—is emerging, heralding the opportunity to transform cancer screening, potentially detecting many more cancers much earlier, saving lives and potentially generating significant economic benefits.
MCED technologies hold the potential to detect, from a simple blood draw, as many as 50 different types of cancers with a very high rate of accuracy, a low false positive rate, and the ability to trace the detected cancer to its likely tissue of origin with a high degree of confidence. As all human cells shed nucleic acid fragments into the bloodstream, the technology works by detecting circulating tumor DNA (ctDNA) in the bloodstream as a biomarker for cancer detection. Further, the technology can link detected cancers to their tissue of origin by analyzing methylation patterns. Methylation refers to an essential step in the process of cellular differentiation—what directs a cell to evolve into kidney, liver, or heart tissue, for instance. With each cell type in the body having a unique methylation pattern, or “fingerprint,” MCED uses a combination of novel biochemistry and AI techniques to learn to connect detected cancers to their likely tissue of origin.
MCED tests can simultaneously screen for signals of dozens of cancers—the vast preponderance of which currently enjoy no effective screening technique—with a potentially high degree of accuracy, opening a new frontier of cancer screening possibility. As Dr. Chetan Bettegowda of Johns Hopkins University explains, MCED introduces the potential “to transform the concept of cancer screening from an organ-by-organ, site-by-site, to a whole system, patient, individual approach.”[33] It also introduces the possibility to screen for cancers in the asymptomatic population. This matters greatly, for, as Dr. Norman “Ned” Sharpless, former director of the National Cancer Institute at the National Institutes of Health, notes, “The most common way people are diagnosed with a cancer is when they present to a doctor with a new symptom [subsequently identified as cancer].”[34] Of these, the vast majority are discovered at the latest stage, stage IV. In fact, out of every 100,000 cancers diagnosed in clinical settings, stage IV cancers account for approximately 170, compared with 100 for stage III, 75 for stage II, and 50 for stage I.[35]
The Importance of Early Cancer Detection
Earlier cancer detection generates significant health and economic benefits, as the following sections explain.
Health Benefits
Cancer is most effectively and efficiently treated when it is caught early, when it is localized, and before it has metastasized to distant parts of the body. As one report explains, “Survival rates improve dramatically when cancer is diagnosed early and the disease is confined to the organ of origin before it has had a chance to spread, and the cancer is more likely to be treated successfully.”[36] Early detection, especially resulting from effective cancer screening protocols, is paramount to reducing mortality from cancer. As the American Cancer Society explains, “Screening is known to reduce mortality for cancers of the breast, colon, rectum, cervix, lung (among current or former heavy smokers), and probably prostate.”[37] On average, catching cancer earlier gives patients a four times greater chance of survival.[38] For instance, one study found that when cancer is diagnosed after it has spread, the five-year cancer-specific survival rate is 21 percent, compared with 89 percent when the cancer is diagnosed early and still localized.[39]
According to a study by Clarke et al., “Projected Reductions in Absolute Cancer–Related Deaths from Diagnosing Cancers Before Metastasis, 2006–2015,” detecting cancers with distant metastases at earlier stages could potentially reduce cancer-related five-year mortality by at least 15 to 24 percent.[40] The study found that detection of multiple cancer types earlier than stage IV could reduce at least 15 percent of cancer-related deaths within five years, affecting not only cancer-specific but all-cause mortality. Stage IV cancers represented 18 percent of all estimated diagnoses but 48 percent of all estimated cancer-related deaths within five years. Assuming all stage IV cancers were diagnosed at stage III, 51 fewer cancer-related deaths would be expected per 100,000, a reduction of 15 percent of all cancer-related deaths. Assuming one-third of metastatic cancers were diagnosed at stage III, one-third diagnosed at stage II, and one-third diagnosed at stage I, 81 fewer cancer-related deaths would be expected per 100,000, a reduction of 24 percent of all cancer-related deaths.[41]
The importance of early detection becomes even clearer when examining its impact on survival rates for certain forms of cancer. Well more than 90 percent of women diagnosed with breast cancer at the earliest stage survive their disease for at least five years, compared with about 15 percent for women diagnosed with the most-advanced stage of disease. More than 80 percent of lung cancer patients will survive for at least one year if diagnosed at the earliest stage, compared with around 15 percent for those diagnosed with the most-advanced stages of the disease. Unfortunately, only about 15 percent of lung cancers are diagnosed at the localized stage, when clinical intervention can markedly improve patient outcomes.[42] Ninety percent of women diagnosed with earliest-stage ovarian cancer survive their disease for at least five years, compared with around 5 percent for women diagnosed with the most-advanced stage of disease. And more than nine in ten bowel cancer patients will survive the disease for more than five years if diagnosed at the earliest stage.[43]
Earlier detection makes all forms of cancer intervention more effective than when cancers are diagnosed at later stages. As Dr. Vogelstein notes, patients with stage III colorectal cancer, if they have micro metastases (i.e., a very small micro-metastatic disease, even if already spread to other organs), given chemotherapy, can recover almost 50 percent of the time; whereas if the cancer becomes visible and bulky (visible metastases), the recovery rate is close to nil. This also holds true for the newest, most cutting-edge interventions, such as targeted immunotherapies (i.e., immune checkpoint inhibitors) and CAR-T-based (chimeric-antigen receptor T cell) therapies: Recovery rates are far higher in patients with low tumor burdens than with high.[44] Indeed, in quite many cases, these technologies mean that patients with localized (i.e., Stage I-II) solid tumors are potentially curable. As Raza writes (about what this evidence makes clear):
What we need now is a paradigm shift. Today, the newest methods generating the most research and expense tend to be focused on treating the worst cases—chasing after the last cancer cells in end-stage patients whose prognoses are the worst. We need instead to commit to anticipating, finding, and destroying the first cancer cells.[45]
Indeed, effective screening can deliver tremendous benefits: Since the pap smear test was introduced, the cervical cancer death rate in the United States has declined by about 70 percent.[46] The first U.S. trial of breast-cancer screening, launched in 1963, reduced mortality by 25 percent in its first 18 years.[47] And analysts estimate that, since 1998, the number of U.S. breast cancer deaths prevented due to mammography increased from 384,000 to 614,000.[48]
According to a 2016 study by Seabury et al., “Quantifying the Gains in the War on Cancer Due to Improved Treatment and Earlier Detection,” an examination of the 15 most-common types of cancers found that the three-year cancer-related mortality of cancer patients fell by 16.7 percent from 1997 to 2007, with advances in early detection responsible for 4.5 percentage points of that decline (in other words, 27 percent of the decline) and advancements in treatment for a reduction of 12.2 percentage points.[49] As the authors wrote, “Cancer detection has seen significant breakthroughs, such as digital mammograms and the development of genetic profile tests.”[50]
Their study found that the relative importance of treatment and detection in reducing mortality varied across cancer types. Improvements in detection contributed to reduced mortality rates for all 15 types of cancers studied, but were most significant for thyroid, prostate, and kidney cancer.[51] Improved early detection accounted for 60 percent of the reduction in the three-year mortality rate for prostate cancer and just about half the reduction for kidney and renal pelvis cancers.[52] Earlier detection of colorectal cancer accounted for 42 percent of the gain in colorectal cancer survival rates from 1997 to 2007, abetted by the fact that the percentage of adults receiving recommended screening for colorectal cancer rose from 44 to 65 percent from 2000 to 2010. (That figure stood at 68.8 percent as of 2018.)[53] Overall, the study estimates that the benefits of earlier detection (for this suite of 15 cancers) during the years 1997 to 2007 generated $19 billion in societal value (even without considering the benefits of having identified patients before they developed malignancies).[54]
Economic Benefits
Earlier and better screening yields economic benefits as well. As noted, cancer screenings have already saved the United States at least $6.5 trillion.[55] Cancer is also much more economical to treat when caught at an earlier as opposed to a later stage. For instance, as noted, in the Medicare population, average total annual costs of care are up to seven times higher for Medicare beneficiaries who are diagnosed in later stages rather than earlier stages.
The 2018 report “Medical Care Costs Associated With Cancer in Integrated Delivery Systems” examined the costs associated with treating cancer from January 1, 2000 to December 31, 2008 in a population of over 45,000 patients diagnosed with one of the four most-commonly diagnosed cancers in the United States (breast, colorectal, lung, and prostate) who were members of one of the four health care plans within the Cancer Research Network. The report shows significant potential economic savings from earlier cancer detection, and that mean total one-year costs for lung cancer ranged from $50,700 (stage I) to $97,400 (stage IV) among patients ages <65 years and from $44,000 (stage I) to $71,200 (stage IV) among patients ages ≥65 years. For colorectal cancer patients under age 65, the five-year cost of treatment for a patient with stage IV cancer was $205,100, compared with $65,000 for a stage I patient. (For individuals over 65 diagnosed with colorectal cancer, the five-year total costs ranged from $67,900 for a stage I patient to $141,000 for stage II patients). Similar trends were apparent for lung and prostate cancer, with the five-year total costs for a stage I lung cancer patient under the age of 65 estimated at $93,800 and for a stage IV patient at $200,300; for prostate cancer, five-year total costs for the under-65 stage I prostate patient stood at $51,800 compared with $72,300 for a stage IV patient.[56]
Overall, the report observes “higher costs among patients diagnosed with advanced versus earlier-stage disease in the fee-for-service setting.” It concludes by noting that “net costs of care were highest for patients aged <65 years with advanced-stage cancers, suggesting that early detection and prevention strategies are key to curtailing high long-term costs associated with late-stage disease.” The report “emphasizes the need for continued effective cancer screening” especially “to reduce the number of invasive colorectal and late-stage female breast cancer diagnoses.”[57] The study’s message is clear: Earlier detection of cancers saves both lives and costs for health care systems and economies more broadly.
Similarly, a 2017 study, “Estimating Cost Savings for Early Cancer Diagnosis,” sought to examine the cost savings from early cancer diagnosis for 19 cancers, assuming that all stage III and IV cases were detected at stage I or II instead (using current incidence rates for these cancers). As the report notes, “In many cases, it is much less costly to treat cancer when it is diagnosed earlier.”[58] In part, that’s because cancer patients’ costs of care in the last year of life are sizably higher than during early stages. The study concluded that earlier diagnosis of those cancers could generate $26 billion in cost savings annually, equivalent to 17 percent of total estimated yearly expenditures on cancer treatment.[59] For breast, lung, prostate, and colorectal cancers, and melanoma, which are the top-five cancers in the United States by incidence, with an estimated 859,110 new cases in 2017 (accounting for 50.9 percent of the 1,688,780 cancer cases diagnosed that year), the study estimated $10.7 billion in savings from earlier diagnosis (about 41.5 percent of cost savings from all cancers).[60]
Those findings also concord with a 2016 study which examined the cost of breast cancer coverage across various stages of the disease. The study concluded that “the costs were higher for patients whose cancer was more advanced at diagnosis, for all cumulative 6-month periods (months 0–6, 0–12, 0–18, and 0–24).” It found that the average costs per patient (as allowed by insurance companies) in the year after diagnosis were $60,637, $82,121, $129,387, and $134,682 for disease stage 0, I/II, III, and IV, respectively, and the costs allowed per patient in the 24 months after the index diagnosis were $71,909, $97,066, $159,442, and $182,655, for those four stages. As the study concluded, “The cost difference based on the stage at diagnosis was largely driven by the cost of chemotherapy and noncancer treatments.”[61] Across the broader U.S. health care system, treatment of metastatic cancer may be as much as two times more costly than treatment of cancer before it metastasizes.[62]
Specific Benefits of MCED Screening Technologies
In terms of cancer screening and detection, MCED approaches are poised to yield several additional unique benefits, beyond the ability to screen for many more cancers and detect them earlier.
First, the simple reality is that it would be logistically, economically, and feasibly impossible to screen individually for up to 50 different cancers. Not only would it be extremely time consuming and expensive to ask patients to take so many individual tests, it would be extremely difficult to logistically administer so many tests. There could also be an accumulation of false positive returns. It’s far more efficient to test for dozens of cancers simultaneously.
Second, the screening process (beyond the blood draw) is much less invasive and, with many cancers presenting as unspecified pains in certain parts of the body, which can even be difficult to physically biopsy, MCED can expand the range of cancers for which earlier detection is physiologically possible.
Third, the logistical ease of MCED testing (from a blood draw) could benefit those living in rural communities who may experience more difficulty in seeing doctors for physical screening exams or more difficulty in accessing specialized screening services. This could have a particularly important impact in ameliorating racial and socioeconomic disparities in cancer screening availability. This matters, especially when cancer deaths in America’s rural areas are 14 percent higher than in urban areas (with that disparity increasing over time). Moreover, the five-year cancer survival rate (for all cancers) in America’s rural areas is 8 percent lower than in urban areas.[63]
In part due to America’s urban/rural divide, the extent of cancer screening also varies considerably by state. For instance, the percentage of adults ages 50 to 75 who reported being up to date with colorectal cancer screening in 2016 ranged from 75.3 percent in Massachusetts to 59.9 percent in Mississippi.[64] Likewise, the percentage of female Medicare enrollees ages 65 to 74 that received an annual mammography screening in 2017 was 39 percent in Mississippi, compared with 54 percent in Massachusetts.[65]
To be sure, there’s a clear distinction between differences in the medical availability of cancer screenings across states and differences in patients’ partaking of those screenings across states. However, as Otis Brawley, Bloomberg distinguished professor of Oncology and Epidemiology at Johns Hopkins University, has noted, many of the disparities in the differences among states in death rates from cancer pertain to socioeconomic factors, such as access to transportation, access to doctors and medical facilities, and affordability of care. In fact, he notes that for women diagnosed with breast cancer, the fatality rate is half in Massachusetts what it is in Mississippi.[66]
A fourth area where MCED approaches could deliver unique patient benefits pertains to equity, in terms of cancer screenings and, potentially, end results. Again, making it logistically and economically feasible to screen for a far greater number of cancers could generate significant equity benefits. That matters especially when Black, American Indian, and Native American individuals face disproportionately higher incidences of cancer fatalities.[67] Black men have the highest overall cancer death rate, which is 19 percent higher than that of White men in the United States.[68] As of 2020, African Americans still have the highest mortality rates of any racial or ethnic group for most cancers. And, while Hispanic men and women are less likely to be diagnosed with cancer than non-Hispanic whites overall, cancer is the leading cause of death among Hispanic Americans.[69]
Certainly there exist a wide range of factors that inform and cause racial, geographic, and socioeconomic disparities in cancer screening rates and in U.S. cancer fatalities. However, differences in access to cancer screening do appear to be one significant causal factor.
Lastly, another benefit of MCED screening tests is that they may be able to help identify patient populations at earlier stages of disease progression, which can provide a platform for companies to innovate therapeutics against. One reason it has been difficult to develop treatments for certain cancers, such as pancreatic cancer, is that they are very difficult to diagnose at an early stage, and as such it’s difficult to identify patient populations against which clinical trials for possible therapeutics can be run. If MCED tests were deployed more widely across the population, and prove to deliver on their potential to identify cancers emerging at earlier stages, they could contribute to the identification of populations in which clinical trials of innovative therapeutics could occur. To be sure, the developers of MCED tests are focused on screening and diagnostics and are not making claims that their technologies can predict disease onset or identify biomarkers. But nevertheless, the point stands that a greater base of knowledge about patient populations with certain cancers at certain stages can provide a further platform on which innovation can occur to address some of these diseases, especially ones for which there are currently no effective treatments. In this regard, patients individually, and society broadly, need more data, not less, about the prevalence of cancer.
Addressing Potential Misgivings About MCED Approaches
While there exist potentially significant benefits from MCED screening, some concerns have been raised, including that they might lead to overdiagnosis or return very high false positive rates.
The first concern pertains to MCED’s potential to detect “indolent” cancers, which are those that progress slowly and do not pose an imminent threat to a patient’s health. Some have called these the types or stages of cancers that people will increasingly die “with,” rather than “from.” As such it’s important that MCED technologies be attuned (or optimized) to identifying particularly the invasive, fastest-growing, most-lethal cancers. In this regard, it’s fortuitous that scientific research appears to indicate that indolent, less-aggressive cancers are less likely to shed ctDNA into the bloodstream and that it tends to be the more-aggressive, faster-growing cancers that are shedding the most ctDNA, conferring better ability for MCED tests to discriminate between indolent and aggressive cancers.[70]
Closely related to the challenge of minimizing overdiagnosis is minimizing the return of false positives. While this will certainly be navigated in MCED screening, the reality is that it’s a challenge with the current single cancer-screening paradigm already, a challenge which we accept considering the vast benefits of current standard-of-care screening. The $27 billion America spends on cancer screening yields about 9 million positive results annually; of these, only 204,000 turn out to be actual cancers, while 8.8 million being false positives.[71] Likewise, the cumulative screening tests recommended for a 60-year-old U.S. female with a history of smoking (thus suggesting screening for breast, colon, cervical, and lung cancers) yield a 37 percent likelihood of at least one false positive result.[72] Critical here are cancer screening tests’ positive predictive value (PPV), which represents the probability that a patient with a positive (abnormal) test result actually has the disease. Here, given a very low comparative false-positive rate, MCED tests are significantly higher than single-cancer screening tests in terms of their PPV.
To be sure, optimizing MCED systems to avoid “overdiagnosis,” increase their PPVs, and lower false positives will be crucially important to the overall success of MCED screening tests as they seek to complement the current standard of care. However, it is important to note that the bigger problem today is underdiagnosis, and the potential to make quite significant progress in that area should not be sacrificed out of fears of overdiagnosis, which the scientific evidence to date suggests can be mitigated by MCED technologies, especially as they continually learn and evolve over time, which is likely to further improve their accuracy. This is a result of intentional test design, and represents the difference in screening strategies regarding how a multi-cancer test can complement standard-of-care single-cancer screening tests.
An Emerging and Intensely Competitive Global Industry
The global competition for leadership in the field of blood-based multi-cancer early detection has become increasingly fierce. No government has embraced MCED more than China.[73] China’s government views the technology both as a growth industry and the answer to China’s own burgeoning cancer burden (where the country encounters 4.8 million new cancer cases and 2.57 million cancer deaths annually).[74] Indeed, a number of innovative, highly competitive Chinese MCED players have emerged, including BurningRock Dx, SeekIn, Berry Oncology, Singlera Genomics, and GeneSeeq.
China’s MCED competitors have leveraged the country’s strengths in genome sequencing—the country now being the world’s leader in genome sequencing capacity—which has given Chinese companies the ability to become increasingly viable players in the field of precision diagnostics and development of accurate, cost-effective, DNA-related diagnostic tests.[75] Moreover, China’s national biotech strategy affords MCED developers access to low-cost state capital, academic biobanks, and other resources. China has also promised to add several precision drugs and molecular-diagnosis products to its national medical-insurance list, ensuring that companies’ research costs will be recouped if they lead to a product.[76] China is doing so because it understands that committing the government as a procurer of such early detection technologies will help build companies and markets.
China’s push toward MCED leadership fits as a part of China’s overall effort to become a much more significant player in the global biopharmaceutical industry, and in the oncology sector in particular.[77] Indeed, China’s share of global drugs under development has increased from just 1 percent in 2005 to 12 percent in 2020, while its share of all oncology drugs under development spiked from just 2 percent to 18 percent over that timeframe. China has risen to the second place in the world in terms of new drug development, with domestic drugs under research accounting for over one-fifth of the global total.[78] Meanwhile, China’s share of value added in the global biopharmaceutical industry jumped four-fold from 2002 to 2019, from a meager 5.6 to robust 24 percent.[79]
Other countries are also looking to ensure their citizens receive the benefits of these technologies first. For instance, in November 2020, the United Kingdom’s National Health Service partnered with GRAIL to test its MCED technology, Galleri, in a program involving 165,000 British citizens, which may be expanded to one million British citizens.
Getting the Regulatory and Coverage Environment Right for MCED in the United States
If the promise of MCED is to be realized in the United States, policymakers will need to get the regulatory and coverage environment right. As medical science’s understanding of cancer has evolved over the past half century, so too has the evolution of recommended cancer screening guidelines. As noted, individuals over 65 account for 60 percent of newly diagnosed malignancies and 70 percent of all cancer deaths in the United States.[80] That makes cancer screening an especially important issue for Americans on Medicare—and that’s why it’s important that Medicare carefully review potentially offering MCED screening as a covered benefit.
Launched in 1966, Medicare is a program administered by the Centers for Medicare and Medicaid Services that helps pay for health care services for citizens over the age of 65, certain younger people with disabilities or chronic diseases, and individuals with end-stage renal disease.[81] When Medicare was launched, it initially covered only acute health care situations (e.g., sicknesses or hospitalizations). However, over time, Medicare has added a range of covered benefits (within specified patient parameters) for preventive services, such as for cardiovascular disease (i.e., Medicare Part B covers cardiovascular screening blood tests once every five years), diabetes (up to two screenings per year), hepatitis C, and HIV.[82] Today, in terms of cancer, Medicare provides covered screening benefits for cervical and vaginal cancer, colorectal cancer, lung cancer (low-dose computerized tomography, or LDCT, once each year), mammograms, and prostate cancer.
However, Medicare does not cover cancer screening as a preventive benefit except where Congress has explicitly amended Medicare laws to provide such coverage. Cancer screening was for the first time added as a statutorily covered Medicare benefit when the Omnibus Budget Reconciliation Act (OBRA) of 1989 added coverage for pap smear tests (and pelvic exams) to examine for cervical and vaginal cancers. A year later, OBRA of 1990 added a benefit for mammography to examine for breast cancer. Specifically Section 4163 of the 1990 OBRA explicitly added coverage for “screening mammography,” defined as “a radiologic procedure provided to a woman for the purpose of early detection of breast cancer.” Importantly, each of these covered benefits applies only to the modality specified, such that Medicare provides a covered benefit for mammograms (i.e., a radiological procedure) but not for other potential modalities of breast cancer screening.
Seven years elapsed before Congress again expanded statutorily covered cancer screening benefits in Medicare, with the Balanced Budget Act of 1997 adding prostate and colorectal cancer screening benefits.[83] Importantly, instead of specifying a modality for colorectal cancer screening, the legislation was crafted to provide flexibility in terms of the types of colorectal cancer screening tests covered, including:
(A) Screening fecal-occult blood cancer test; (B) Screening flexible sigmoidoscopy; (C) In the case of an individual at high risk for colorectal cancer, screening colonoscopy; (D) Such other tests or procedures, and modifications to tests and procedures under this subsection, with such frequency and payment limits, as the Secretary determines appropriate, in consultation with appropriate organizations.[84]
The expansiveness of the covered benefit for colorectal screenings has proven to be prescient, for today it allows for Medicare to provide benefits for colorectal cancer screening modalities that didn’t exist in the late 1990s. For instance, home-based stool DNA tests such as Exact Sciences’ Cologuard can detect microscopic amounts of blood in stool samples and check for certain DNA changes and mutations found in cancerous tumors or precancerous polyps, indicating the potential presence of colon cancer. Recognizing that DNA changes and mutations may differ between colon cancers, the stool DNA test targets multiple DNA markers, which has helped other such tests achieve high detection rates of early-stage colon cancer.[85]
In other words, by not limiting itself to particular modalities, the 1997 Balanced Budget Act provided a constructive pathway for innovative colorectal cancer screening approaches to emerge in future years and receive Medicare coverage. Since Congress created the Medicare coverage provision for colon cancer screening in 1997, it has subsequently spawned a wave of innovation in the field, with today many more colon-cancer screening tests available to patients and the percentage of Americans (at the recommended screening ages) taking colon cancer tests now about 50 percent.
Multi-Cancer Early Detection Policy Recommendations
As a new generation of multi-cancer early detection technologies are coming to the fore, it’s time for Congress to act again, by creating a pathway for Medicare coverage of MCED screening tests.[86] And that’s exactly what bipartisan, bicameral legislation in the “Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act” seeks to do. The Act addresses the misalignment between advances in science and Medicare coverage by permitting Medicare coverage of multi-cancer screening. The legislation creates the authority for the Centers for Medicare & Medicaid Services (CMS) to use an evidence-based process to cover blood-based MCED tests and future test methods once approved by the U.S. Food and Drug Administration, while maintaining CMS’s authority to use an evidence-based process to determine coverage parameters for these new types of tests. The legislation would ensure Medicare beneficiaries are eligible to benefit on a timely basis from MCED screening technologies. The legislation enjoys broad bipartisan support, sponsored by 295 members of the U.S. House of Representatives and 62 U.S. Senators.[87]
Cancer has proven to be a relentless and wily enemy. To defeat it, we’re going to need to embrace equally creative solutions and radically new approaches, such as how checkpoint blockading and immunotherapy transformed cancer therapy. MCED heralds the potential for another significant breakthrough to dramatically enhance patients’ lives by detecting cancers earlier, and to put the nation’s cancer response on a more-sustainable economic footing. It’s time for policymakers to embrace this opportunity.
Supporting Innovation in Breakthrough Medical Products
The “Ensuring Patient Access to Critical Breakthrough Products Act of 2025” would help ensure prompter coverage of breakthrough devices under Medicare, addressing a long-standing barrier in U.S. healthcare innovation: the delay between FDA approval and Medicare coverage. Such regulatory and reimbursement lags weaken the incentives for firms to invest in high-risk, high-cost devices, since even successful FDA approval may not translate into timely patient access and revenue.
By establishing a transitional coverage period for devices designated as “breakthrough” by the FDA, the Act can shorten the time between invention and adoption. This ensures patients benefit sooner from life-saving technologies while strengthening firms’ incentives to continue investing in innovation. The policy is especially timely given recent FDA staffing reductions that have slowed device reviews, and studies showing median review times for high-risk therapeutic medical devices of 0.96 years (11.5 months) under the FDA’s premarket approval pathway.[88]
By guaranteeing temporary Medicare coverage, the Act reduces uncertainty surrounding reimbursement timing, giving firms and investors more predictable returns in the interim period. This predictability allows companies to make long-term capital deployment decisions with greater confidence, knowing that Medicare will not be a bottleneck once the FDA has granted breakthrough designation.
ITIF has previously warned against policies that have undermined U.S. medical device competitiveness, such as excise taxes on the sales of medical devices and unclear patent subject matter eligibility requirements.[89] In fact, one recent study found that although 17,743 patent applications for medical devices and diagnostics were rejected in the United States as ineligible for patent protection, 1,694 of those were granted by the European Patent Office, by China’s patent office, or both.[90] The “Ensuring Patient Access to Critical Breakthrough Products Act of 2025” moves in the opposite direction: it lowers barriers, strengthens incentives to innovate, and ensures that Medicare beneficiaries and the broader economy gain from a more competitive medical device industry.
Conclusion
ITIF commends Congress for examining how emerging technologies and supportive policies can enhance American seniors’ access to breakthrough medical care.
Thank you for your consideration.
Endnotes
[1]. American Cancer Society (ACS), “American Cancer Society Releases Latest Global Cancer Statistics; Cancer Cases Expected to Rise to 35 Million Worldwide by 2050,” April 4, 2024, https://pressroom.cancer.org/GlobalCancerStatistics2024.
[2]. World Health Organization, “Early cancer diagnosis saves lives, cuts treatment costs,” news release, February 3, 2017, https://www.who.int/news/item/03-02-2017-early-cancer-diagnosis-saves-lives-cuts-treatment-costs.
[3]. American Cancer Society, “All About Cancer,” https://www.cancer.org/cancer.html.
[4]. Rebecca L. Siegel, “Cancer statistics, 2024” CA: A Cancer Journal for Physicians Vol. 74, Issue 1 (January/February 2024): 12-49, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21820.
[5]. American Cancer Society, “Cancer Facts and Figures, 2025,” (ACS, 2025), 1, https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2025/2025-cancer-facts-and-figures-acs.pdf.
[6]. National Cancer Institute, “Age and Cancer Risk,” May 2, 2025, https://www.cancer.gov/about-cancer/causes-prevention/risk/age.
[7]. Ibid.
[8]. American Cancer Society, “Cancer in Medicare: An American Cancer Society Cancer Action Network Chartbook,” January 23, 2024, https://www.fightcancer.org/policy-resources/cancer-medicare-american-cancer-society-cancer-action-network-chartbook.
[9]. Azra Raza, “Cancer is Still Beating Us—We Need a New Start,” The Wall Street Journal, October 4, 2019, https://www.wsj.com/articles/cancer-is-still-beating-uswe-need-a-new-start-11570206319.
[10]. “Deaths by cancer in the U.S. from 1950 to 2017 (per 100,000 population),” Statista, https://www.statista.com/statistics/184566/deaths-by-cancer-in-the-us-since-1950/.
[11]. Rebecca L. Siegel, Kimberly D. Miller, and Ahmedin Jemal, “Cancer statistics, 2019,” CA: A Cancer Journal for Clinicians Vol. 69, Issue 1, (2019): 7–34, https://onlinelibrary.wiley.com/doi/full/10.3322/caac.21551.
[12]. American Cancer Society, “Cancer Facts & Figures 2020,” 1.
[13]. Susan G. Komen Foundation, “The Who, What, Where, When and Sometimes, Why.,” https://www.komen.org/breast-cancer/facts-statistics/survival-rates/; Monique Ellis, “The most successful cancer drugs in 2019,” Proclinical, March 13, 2019, https://www.proclinical.com/blogs/2019-3/the-most-successful-cancer-drugs-in-2019.
[14]. Seth A. Seabury et al., “Quantifying Gains in the War on Cancer Due to Improved Treatment and Earlier Detection,” Forum for Health Economics & Policy, Vol. 19, Issue 1 (November 2015), https://www.degruyter.com/view/journals/fhep/19/1/article-p141.xml.
[15]. Frank R. Lichtenberg, “The Impact of Pharmaceutical Innovation on Premature Cancer Mortality in Canada, 2000-2011” (working paper no. 212139, National Bureau of Economic Research, June 2015), http://www.nber.org/papers/w21239.
[16]. Raza, “Cancer is Still Beating Us—We Need a New Start.”
[17]. Remarks of Dr. Bert Vogelstein, “Webinar on the Multi-Cancer Early Detection Screening Coverage Act” (March 10, 2021), https://tinyurl.com/yhoexce3.
[18]. Raza, “Cancer is Still Beating Us—We Need a New Start.”
[19]. Mingyang Song et al., “Cancer prevention: Molecular and epidemiologic consensus,” Science Vol. 361, Issue 6409 (September 2018): 1317–1318, https://science.sciencemag.org/content/361/6409/1317/tab-pdf.
[20]. Anita Soni, “Top Five Most Costly Conditions among Adults Age 18 and Older, 2012: Estimates for the U.S. Civilian Noninstitutionalized Population,” Statistical Brief #471 (Agency for Healthcare Research and Quality, April 2015), https://www.meps.ahrq.gov/data_files/publications/st471/stat471.pdf.
[21]. National Cancer Institute, “Financial Burden of Cancer Care,” (accessed September 16, 2025), https://progressreport.cancer.gov/after/economic_burden.
[22]. Erica Klinger, “Cancer Costs and Options for Care in the United States,” Association for Accessible Medicines, accessed September 16, 2025, https://accessiblemeds.org/resources/blog/cancer-costs-and-options-care-united-states/.
[23]. Simiao Chen et al., “Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050” JAMA Oncology Vol. 9 Issue 4 (2023): 465-472, doi:10.1001/jamaoncol.2022.7826.
[24]. K. Robin Yabroff, “Estimates and Projections of Value of Life Lost From Cancer Deaths in the United States,” Journal of the National Cancer Institute Vol. 100, Issue 24 (December 17, 2008): 1,755–1,762, https://academic.oup.com/jnci/article/100/24/1755/2606867.
[25]. Mark Fendrick, “Cancer screenings have saved the U.S. at least $6.5 trillion, study estimates,” Institute for Health Policy and Innovation at the University of Michigan, August 17, 2023, https://ihpi.umich.edu/news-events/news/cancer-screenings-have-saved-us-least-65-trillion-study-estimates.
[26]. Kevin Murphy and Robert Topel, “The value of health and longevity,” Journal of Political Economy (2006), 114:871–904, https://www.nber.org/papers/w11405.
[27]. American Cancer Society, “Cancer in Medicare: An American Cancer Society Cancer Action Network Chartbook,” January 23, 2024, https://www.fightcancer.org/policy-resources/cancer-medicare-american-cancer-society-cancer-action-network-chartbook; Andrew Shooshtari, Yamini Kalidindi, and Jeah Jung, “Cancer Care Spending and Use by Site of Provider-Administered Chemotherapy in Medicare,” The American Journal of Managed Care Vol. 25, Issue 6 (June 2019), https://www.ajmc.com/view/cancer-care-spending-and-use-by-site-of-provideradministered-chemotherapy-in-medicare.
[28]. Chen et al., “Estimates and Projections of the Global Economic Cost.”
[29]. American Cancer Society, “Cancer Screening and Prevention in the Medicare Program,” https://www.fightcancer.org/sites/default/files/docs/resources/cancer-screening-and-prevention-in-medicare.pdf.
[30]. U.S. Preventive Services Task Force (USPSTF), “Recommendations,” https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P. Screening includes methods with a USPSTF A, B, or C rating (breast, colon, cervical, prostate, and lung).
[31]. Stephen Ezell, “Seizing the Transformative Opportunity of Multi-cancer Early Detection” (ITIF, April 2021), https://itif.org/publications/2021/04/19/seizing-transformative-opportunity-multi-cancer-early-detection/.
[32]. ACS, “Cancer in Medicare: An American Cancer Society Cancer Action Network Chartbook.”
[33]. U.S. Department of Health and Human Services Food and Drug Administration, Center for Devices and Radiological Health, “Public Workshop: Detecting Circulating Tumor DNA for Cancer Screening” (FDA, March 9, 2020), 19, https://www.fda.gov/medical-devices/workshops-conferences-medical-devices/public-workshop-detecting-circulating-tumor-dna-cancer-screening-03092020-03092020.
[34]. Dr. Norman “Ned” Sharpless, “Remarks at ‘People v. Cancer Summit.”
[35]. Remarks of Hans Bishop, Chief Executive Officer, GRAIL, Inc., “Webinar on the Multi-Cancer Early Detection Screening Coverage Act.”
[36]. Zura Kakushadze, Rakesh Raghubanshi, and Willie Yu, “Estimating Cost Savings from Early Cancer Diagnosis,” Data Vol. 2, Issue 30 (September 2017), https://www.researchgate.net/publication/319475610_Estimating_Cost_Savings_from_Early_Cancer_Diagnosis.
[37]. American Cancer Society, “Cancer Facts & Figures 2020,” 1.
[38]. Eric Young, “New Research Highlights Just One In Seven Diagnosed Cancers Found By A Recommended Screening Test,” news release, December 14, 2022, https://www.norc.org/research/library/new-research-highlights-just-one-in-seven-diagnosed-cancers-foun.html.
[39]. “GRAIL Announces Validation of Its Multi-Cancer Early Detection Test Published in Annals of Oncology,” Biospace, news release, https://www.biospace.com/article/releases/grail-announces-validation-of-its-multi-cancer-early-detection-test-published-in-annals-of-oncology/. Citing: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2018 Sub. Includes persons ages 50–79 diagnosed 2006–2015. “Early/Localized” includes invasive localized tumors that have not spread beyond organ of origin, “Late/Metastasized” includes invasive cancers that have metastasized beyond the organ of origin to other parts of the body.
[40]. Christina A. Clarke, “Projected Reductions in Absolute Cancer–Related Deaths from Diagnosing Cancers Before Metastasis, 2006–2015,” Cancer Epidemiology, Biomarkers, and Prevention Vol. 29, Issue 5 (May 2020), https://cebp.aacrjournals.org/content/29/5/895.
[41]. Ibid.
[42]. Ronald Piana, “The Ongoing Challenges of Lung Cancer Screening,” The ASCO Post, May 25, 2017, https://ascopost.com/issues/may-25-2017/the-ongoing-challenges-of-lung-cancer-screening/.
[43]. Cancer Research UK, “Why is early diagnosis important?” https://www.cancerresearchuk.org/about-cancer/cancer-symptoms/why-is-early-diagnosis-important.
[44]. Remarks of Dr. Bert Vogelstein, “Webinar on the Multi-Cancer Early Detection Screening Coverage Act.”
[45]. Raza, “Cancer is Still Beating Us—We Need a New Start.”
[46]. American Cancer Society, “History of Cancer Screening and Early Detection,” https://www.cancer.org/cancer/cancer-basics/history-of-cancer/cancer-causes-theories-throughout-history11.html.
[47]. Natasha Gilbert, “An uncertain diagnosis,” Nature Vol. 579 (March 26, 2020), https://media.nature.com/original/magazine-assets/d41586-020-00841-8/d41586-020-00841-8.pdf.
[48]. Doug Rix, “Cancer screening; What’s new, what’s coming and what you should consider,” SSWire, February 3, 2020, https://www.swissre.com/reinsurance/life-and-health/reinsurance/cancer-risks/cancer-screening-what-you-should-consider.html.
[49]. Seabury et al., “Quantifying Gains in the War on Cancer Due to Improved Treatment and Earlier Detection.”
[50]. Ibid.
[51]. Ibid., 150.
[52]. Ibid.
[53]. Djenaba A. Joseph, MD et al., “Vital Signs: Colorectal Cancer Screening Test Use–United States, 2018,” Centers for Disease Control and Prevention, https://www.cdc.gov/mmwr/volumes/69/wr/mm6910a1.htm.
[54]. Seabury et al., “Quantifying Gains in the War on Cancer Due to Improved Treatment and Earlier Detection,” 153.
[55]. Mark Fendrick, “Cancer screenings have saved the U.S. at least $6.5 trillion, study estimates,” Institute for Health Policy and Innovation at the University of Michigan, August 17, 2023, https://ihpi.umich.edu/news-events/news/cancer-screenings-have-saved-us-least-65-trillion-study-estimates.
[56]. Banegas et al., “Medical Care Costs Associated With Cancer in Integrated Delivery Systems.”
[57]. Ibid., 408.
[58]. Kakushadze, Raghubanshi, and Yu, “Estimating Cost Savings from Early Cancer Diagnosis,” 2.
[59]. Ibid., 13.
[60]. Ibid.
[61]. Helen Blumen, Kathryn Fitch, and Vincent Polkus, “Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service,” American Health Drug Benefits Vol. 9, Issue 1 (February 2016): 23–32, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4822976/.
[62]. Based on stage II and stage IV breast, colorectal, lung cancer, and metastatic/non-metastatic pancreatic cancer: Banegas et al., “Medical Care Costs Associated With Cancer in Integrated Delivery Systems”; Stacey Costa Byfield et al., “Healthcare costs, treatment patterns, and resource utilization among pancreatic cancer patients in a managed care population” Journal of Medical Economics Vol.16, Issue 12 (December 2013): 1379-1386, https://pubmed.ncbi.nlm.nih.gov/24074258/.
[63]. The National Grange, “New research finds disproportionate impact of cancer on rural communities,” February 2, 2023, https://www.morningagclips.com/new-research-finds-disproportionate-impact-of-cancer-on-rural-communities/.
[64]. Centers for Disease Control and Prevention, “Use of Colorectal Cancer Screening Tests by State,” https://www.cdc.gov/cancer/dcpc/research/articles/use-colorectal-screening-tests-state.htm.
[65]. County Health Rankings and Roadmaps, “Mammography screening” (accessed December 1, 2020), https://www.countyhealthrankings.org/explore-health-rankings/measures-data-sources/county-health-rankings-model/health-factors/clinical-care/quality-of-care/mammography-screening.
[66]. Otis Sharpley, Bloomberg Distinguished Professor of Oncology and Epidemiology, Johns Hopkins University, “Remarks at ‘People v. Cancer Summit” (AtlanticLIVE, November 18, 2020), https://www.youtube.com/watch?v=xIEisUiYNwM.
[67]. American Cancer Society, “Factors Influencing Cancer Disparities,” January 11, 2024, https://www.fightcancer.org/policy-resources/factors-influencing-cancer-disparities.
[68]. Sonya Collins, “2024—First Year the US Expects More than 2M New Cases of Cancer,” American Cancer Society, January 17, 2024, https://www.cancer.org/research/acs-research-news/facts-and-figures-2024.html.
[69]. The National Minority Quality Forum, “National Minority Quality Forum Urges Action in Multi-Cancer Early Detection,” news release, December 4, 2020, https://www.nmqf.org/nmqf-media/mced.
[70]. Oliver Venn et al., “Tumor Shedding into Cell-free DNA (cfDNA) is Associated with High-mortality Cancers,” (poster presented at: The Biology of Genomes Meeting, May 7-11, 2019, Cold Spring Harbor, New York), https://grail.com/wp-content/uploads/2019/05/BOG_2019_Tumor_Fraction_Venn_Poster_Final-1.pdf; A similar statement was made by Dr. Catherine Marinac, “Remarks at ‘People v. Cancer Summit.”
[71]. Ofman and Raza, “Taking Early Cancer Detection to the Next Level.”
[72]. Constance D. Lehman et al., “National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium” Radiology Vol. 283, Issue 1 (December 5, 2016), https://pubs.rsna.org/doi/full/10.1148/radiol.2016161174; U.S. Food and Drug Administration, “PMA P130017: FDA summary of safety and effectiveness data,” August 11, 2014; Joy Melnikow et al., “Screening for Cervical Cancer With High-Risk Human Papillomavirus Testing” Journal of the American Medical Association Vol, 320, Issue 7 (2018): 687-705, https://jamanetwork.com/journals/jama/fullarticle/2697703; Paul F. Pinsky et al., “Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment” Annals of Internal Medicine Vol. 162, Issue 7 (April 2015): 485-491, https://pubmed.ncbi.nlm.nih.gov/25664444/.
[73]. Stephen Ezell, “America Can’t Afford to Lose the Early Cancer Detection Race to China,” Innovation Files, December 9, 2024, https://itif.org/publications/2024/12/09/america-cant-afford-to-lose-the-early-cancer-detection-race-to-china/.
[74]. Changfa Xia et al., “Cancer statistics in China and United States, 2022: profiles, trends, and determinants” Chinese Medical Journal Vol. 135, Issue 5 (February 2022): 584–590, https://pmc.ncbi.nlm.nih.gov/articles/PMC8920425/.
[75]. P. Kirk, “Why China Is Now the World Leader in Genome Sequencing Capacity,” Dark Daily, June 28, 2010, https://www.darkdaily.com/2010/06/28/why-china-is-now-the-world-leader-in-genome-sequencing-capacity-062810/.
[76]. David Cyranoski, “China’s bid to be a DNA superpower” Nature Vol 534 (2016): 462–463, https://www.nature.com/articles/534462a.
[77]. Sandra Barbosu, “How Innovative Is China in Biotechnology?” (ITIF, July 2024), https://itif.org/publications/2024/07/30/how-innovative-is-china-in-biotechnology/.
[78]. “China rises to world’s second place in new drug development,” Xinhua, September 11, 2025, https://english.news.cn/20250911/2d5030b9541e480bb98c77630c92443a/c.html.
[79]. National Science Foundation, Science & Engineering Indicators, Production and Trade of Knowledge- and Technology-Intensive Industries, Table SKTI-9: Value added of pharmaceuticals industry, by region, country, or economy: 2002–19; accessed May 5, 2024, https://ncses.nsf.gov/pubs/nsb20226/data.
[80]. Drishya S. Panikkar, Geeta S. Narayanan, and Kiran Kumar, “Cancer Care in Elderly Patients; A Record Based Retrospective Analysis of Clinical Profile and Overview of Treatment” Asian Pacific Journal of Cancer Biology Vol. 9, No. 2 (2024), https://waocp.com/journal/index.php/apjcb/article/view/1412.
[81]. Medicare.gov, “What is Medicare?” https://www.medicare.gov/what-medicare-covers/your-medicare-coverage-choices/whats-medicare.
[82]. Medicare.gov, “Is my test, item, or service covered?” https://www.medicare.gov/coverage/preventive-screening-services.
[83]. Congressional Research Service, “Medicare Coverage of Clinical Preventive Services” (CRS, March 2010), 8, https://crsreports.congress.gov/product/pdf/R/R40978/6.
[84]. Federal Register, “Balanced Budget Act of 1997,” 42 CFR Part 400, https://omlc.org/news/jan98/BBA1997.txt.
[85]. Colorectal Cancer Alliance, “Stool DNA,” https://www.ccalliance.org/screening-prevention/screening-methods/stool-dna.
[86]. Ezell, “Seizing the Transformative Opportunity of Multi-cancer Early Detection.”
[87]. Congress.gov, “H.R.2407 - Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act,” https://www.congress.gov/bill/118th-congress/house-bill/2407/cosponsors; Congress.gov, “S.2085 - Medicare Multi-Cancer Early Detection Screening Coverage Act,” https://www.congress.gov/bill/118th-congress/senate-bill/2085/cosponsors.
[88]. Todd Shycock, “FDA layoffs delaying medical device reviews,” Medical Economics, March 27, 2025, https://www.medicaleconomics.com/view/fda-layoffs-delaying-medical-device-reviews; Kushal T Kadakia et al., “Market of First Launch for High-Risk Therapeutic Medical Devices,” JAMA Network Open Vol. 7, Issue 12 (December 2024), https://pmc.ncbi.nlm.nih.gov/articles/PMC11624582/.
[89]. Stephen Ezell, “Affordable Care Act Endangers Innovative U.S. Life Sciences Industries,” Innovation Files, July 4, 2013, https://itif.org/publications/2013/07/04/affordable-care-act-endangers-innovative-us-life-sciences-industries/.
[90]. Kevin Madigan and Adam Mossoff, “Turning Lead to Gold: How Patent Eligibility Doctrine Is Undermining U.S. Leadership in Innovation” George Mason Law Review Vol. 24 (April 2017): 939-960, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2943431.


